Chromate / Chromium
Precise rapid tests for water and waste water samples
In the environment, chromium is present only in the form of compounds; the most important natural source is chromite (FeCr2O4). This also serves as starting material for the synthesis of chromium and chromium compounds.
Among other things, chromium is industrially widely used as an alloying element in the field of metal finishing. It is the most important alloying element for the production of stainless and heat-resistant steels. In addition, chromates are used in electroplating (generation of corrosion-resistant chromium coatings), tanneries (suede) and in the paint industry (production of pigment colors).
In industrial wastewaters, trivalent (chromium(III) ions) and hexavalent (chromate and dichromate ions) chromium compounds are found.
Chromates are toxic and carcinogenic. The toxicity of chromium(VI) ions is much higher than that of the chromium(III) ions. However, chromium and its compounds are essential for humans and play a crucial role in the breakdown of glucose in the blood.
Our Chromate/Chromium Products
| Product | Platform | Range | REF | Order | |||||
|---|---|---|---|---|---|---|---|---|---|
| Chromium Test Paper | QT | > 2 mg/L Cr3+ or > 5 mg/L CrO42- | 90724 |
|
|||||
| QUANTOFIX® Chromate | HT | 0 · 3 · 10 · 30 · 100 mg/L CrO42- | 91301 |
|
|||||
| VISOCOLOR® ECO Chromium (VI) | CO | 0.02 · 0.05 · 0.10 · 0.15 · 0.20 · 0.30 · 0.40 · 0.50 mg/L Cr(VI) | 931020 |
|
|||||
| NANOCOLOR® Chromate 5 | TT | 0.1 - 4.0 mg/L CrO42- | 985024 |
|
|||||
| NANOCOLOR® Total Chromium 2 | TT | 0.05 - 2.0 mg/L Cr | 985059 |
|
|||||
| NANOCOLOR® Chromate standard test | ST | 0.01 - 6.0 mg/L CrO42- | 91825 |
|
Reaction basis 🧪
- General: For the determination of total chromium, all oxidation states have to be oxidized to Chromium(VI).
- Colorimetric and photometric determination is carried out in analogy to APHA 3500-Cr D and DIN 38405-D24.
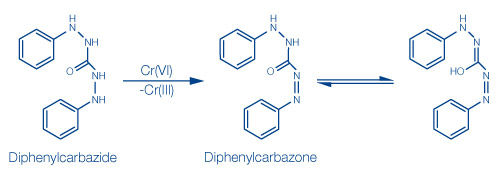
- In sulfuric acid, chromate ions react with diphenylcarbazide to form a purple color. Here, the chromate first oxidizes the diphenylcarbazide(I) to diphenylcarbazone(II). At the same time, chromate (cromium(VI)) is reduced to chromium(III). The enol form of the carbazone forms a purple neutral chelate complex with chromium(III) with the molar ratio of chromium:diphenylcarbazone = 1:1.
Sample preservation 🧪
After adjusting the pH with nitric acid to a value of 1–2, the sample can be preserved for storage for up to 1 month (storage vessel: PE or glass bottle).
Chromate samples must be measured quickly within one day. The sample must be cooled.
Tips & tricks 🧪
- Background information
- Detection of chromium(III) ions requires the prior oxidation to chromium(VI). Without decomposition, only dissolved chromium(VI) compounds are detected. To determine chromium(III), total chromium and chromium(VI) are determined. The difference yields the amount of chromium(III) ions. This calculation can be performed only if there are no insoluble chromium(VI) compounds in the sample solution. These are also determined after decomposition only.
- Sea water suitability
- Almost all VISOCOLOR® and NANOCOLOR® chromate tests are suitable for sea water analysis. The exception is the standard test NANOCOLOR® total Chromium 2 that does not permit sea water analysis. For more information, please refer to the instruction leaflet.
- pH
- The sample solution pH values stated in the instruction leaflets must be complied with. If necessary, adjust the pH with nitric acid or sodium hydroxide.
- Interferences
- Coloration, turbidity and larger amounts of organic substances as well as oxidizing or reducing substances interfere with the determination of the tube test NANOCOLOR® Chromate 5 and the standard test NANOCOLOR® Chromate.
- Chloride interferes with the tube test NANOCOLOR® total Chromium 2 at concentrations above 1000 mg/L.
- Additional interferences are listed in the respective instruction leaflet.
- Turbidity
- Turbid solutions must be filtered prior to the determination of dissolved chromate; turbidity leads to incorrect results: For coarsely dispersed turbidities, use qualitative filter paper (e.g. MN 615), for moderately dispersed turbidities, use glass-fiber paper (e.g. MN 85/70 BF) or or membrane filtration set GF/PET 0.45 μm, for finely dispersed turbidities, use membrane filtration kit 0.45 μm or GF/PET 0.45 μm.

